trending topics
market reports
-

MEDICAL JAPAN 2025 OSAKA Returns to Showcase Global Innovations
2025-02-17
-

Visit MEDICAL JAPAN 2023 TOKYO and take full advantage of the business opportunities!
2023-09-01
-
US to distribute 400 million free N95 masks at CVS, Walgreens in COVID fight
2022-01-21
-

Ethiopia receives additional 2.2 mln doses of Chinese-donated COVID-19 vaccines
2022-01-21
-
Hong Kong researchers say they develop novel material able to kill COVID-19 virus
2022-01-14
-

10 million more Chinese doses on way for Kenya
2022-01-14
-

Sino-African ties on track for a brighter future
2022-01-07
-
Efforts urged to boost COVID-19 vaccine production capacity in poor countries
2022-01-07
-
UAE approves Sinopharm's new protein-based COVID-19 vaccine
2022-01-07
-
UAE approves Sinopharm's new protein-based COVID-19 vaccine
2022-01-07
WHO team assessing Chinese vaccines for emergency use
2021-01-13
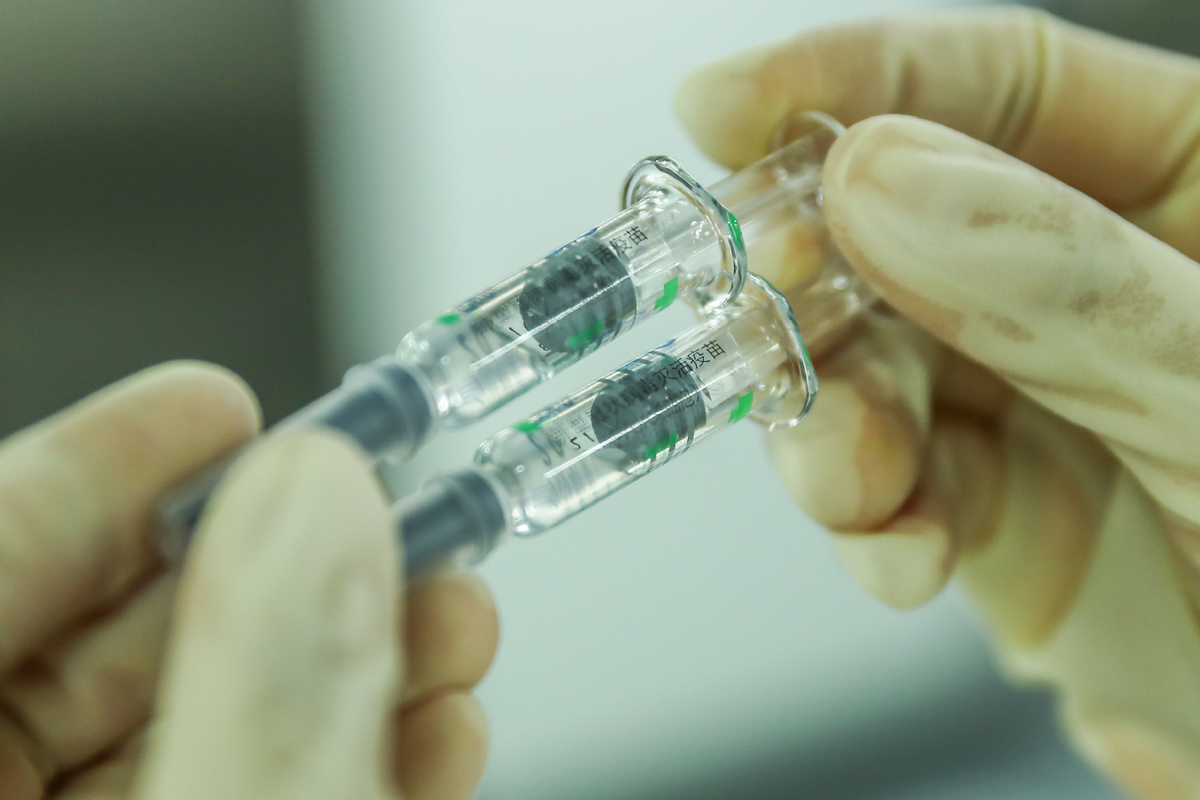
A staff member checks the packages of the COVID-19 inactivated vaccine at Sinovac Biotech Ltd, in Beijing, Dec 25, 2020. [Photo/Xinhua]
A World Health Organization team is in China currently working with producers of COVID-19 vaccines Sinovac and Sinopharm to assess compliance with international quality manufacturing practices ahead of potential emergency use listing by the WHO, said Director-General Tedros Adhanom Ghebreyesus during a press briefing on Monday.
Meanwhile, Tedros urged COVID-19 vaccine manufacturers from around the world to move swiftly to provide the necessary data which will allow the organization to consider them for emergency use listings.
The team is separate from the WHO mission which will arrive in China on Thursday to conduct joint scientific research with Chinese peers on the origin of the novel coronavirus, according to the WHO chief.
A number of countries, including Egypt, the UAE, Jordan and Indonesia, have authorized the COVID-19 vaccines produced by China for emergency use. And many more countries, including Brazil, Chile, Malaysia, the Philippines, Thailand and Nigeria, have ordered Chinese vaccines or are cooperating with China in procuring or rolling out the vaccines.
More than 9 million doses of homegrown coronavirus vaccine have been administered in China, the National Health Commission said on Saturday.
(China Daily)



 My Member
My Member Message Center
Message Center