

FOB Price : Get a Price/Quote
Min.Order : 1000 Piece(s)
Certification : CE,CFS
Brand Name : INVBIO
Payment Terms : T/T
brand name : INVBIO
certification : CE,CFS
min.order : 1000 Piece(s)
warranty : 24 months
payment terms : T/T
Packaging : 20 test per box
Specification : 1000 boxes per carton
place of origin : Beijing
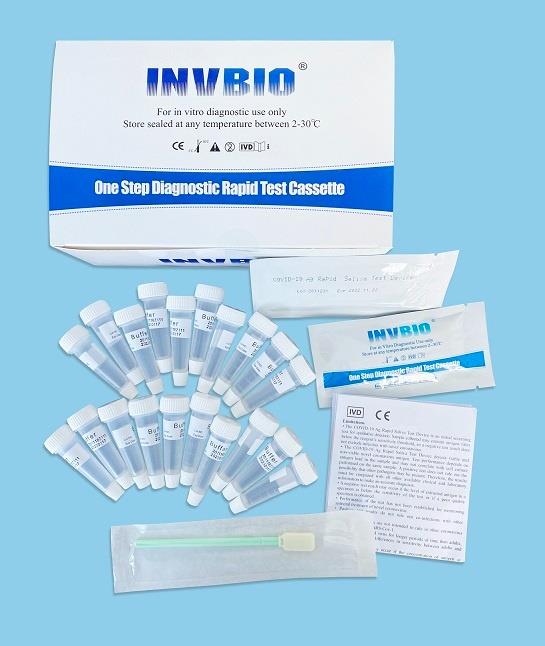
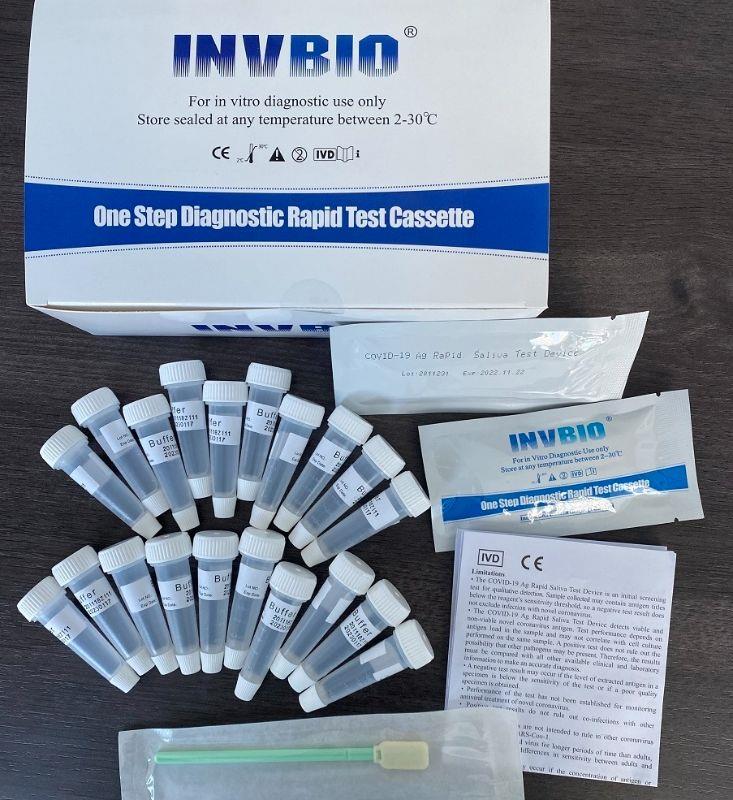
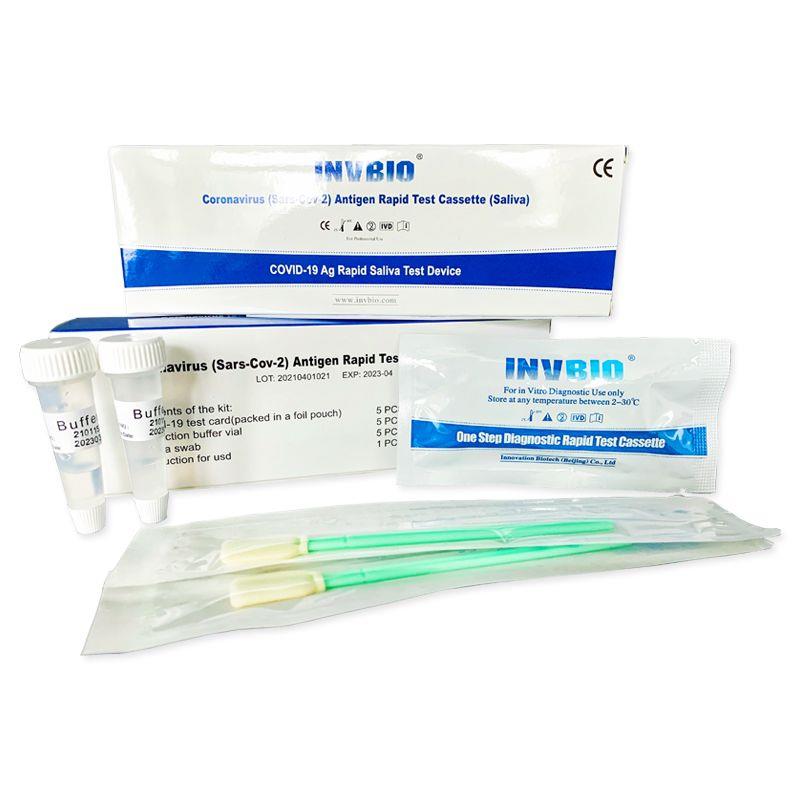
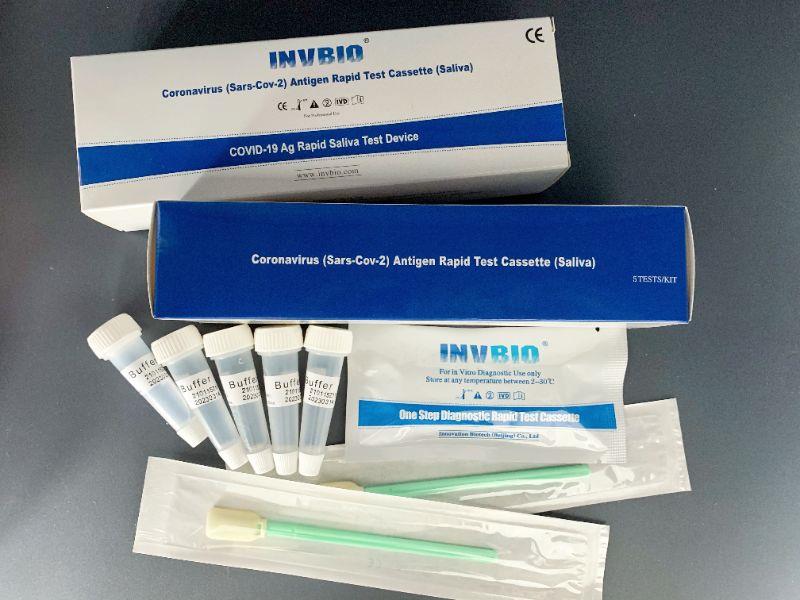
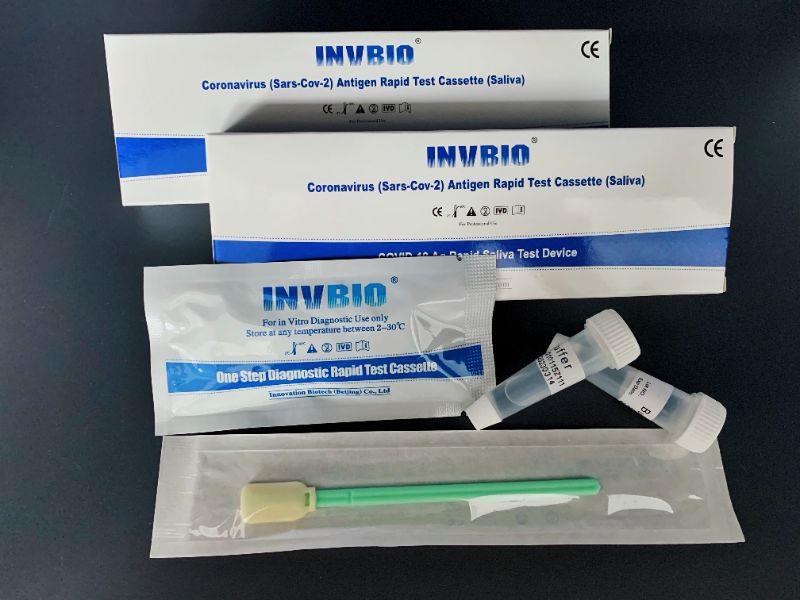
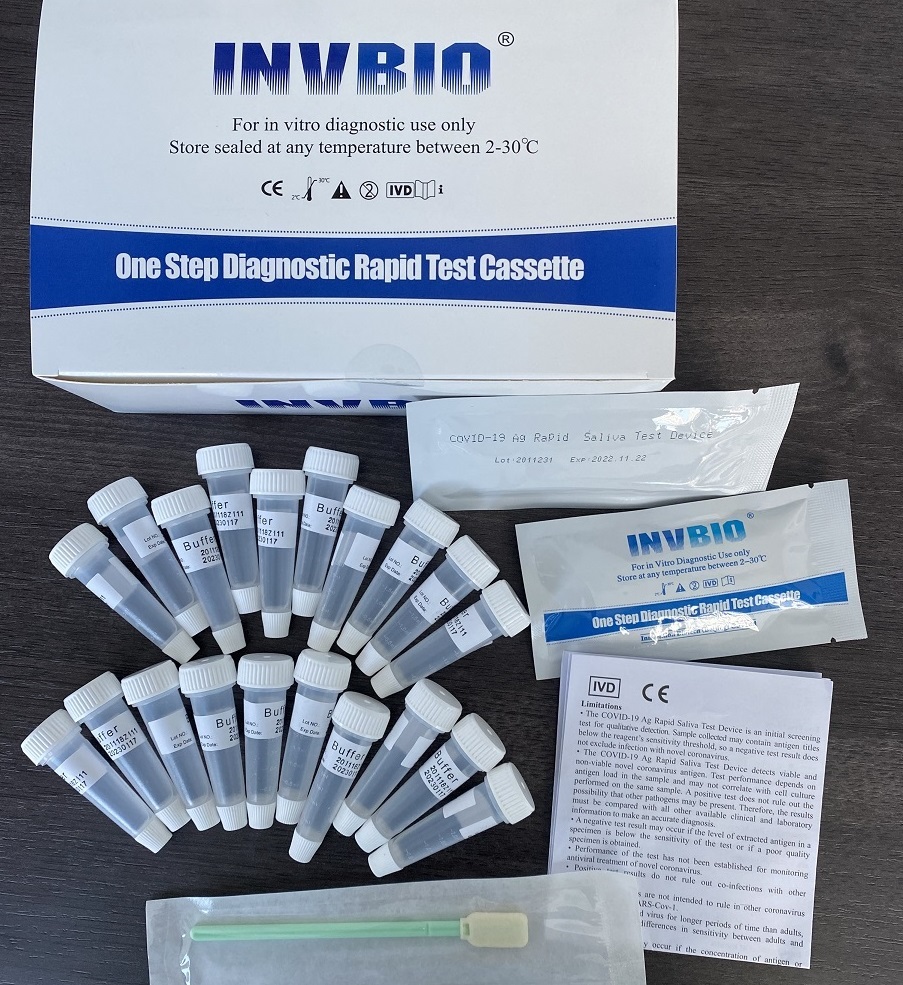
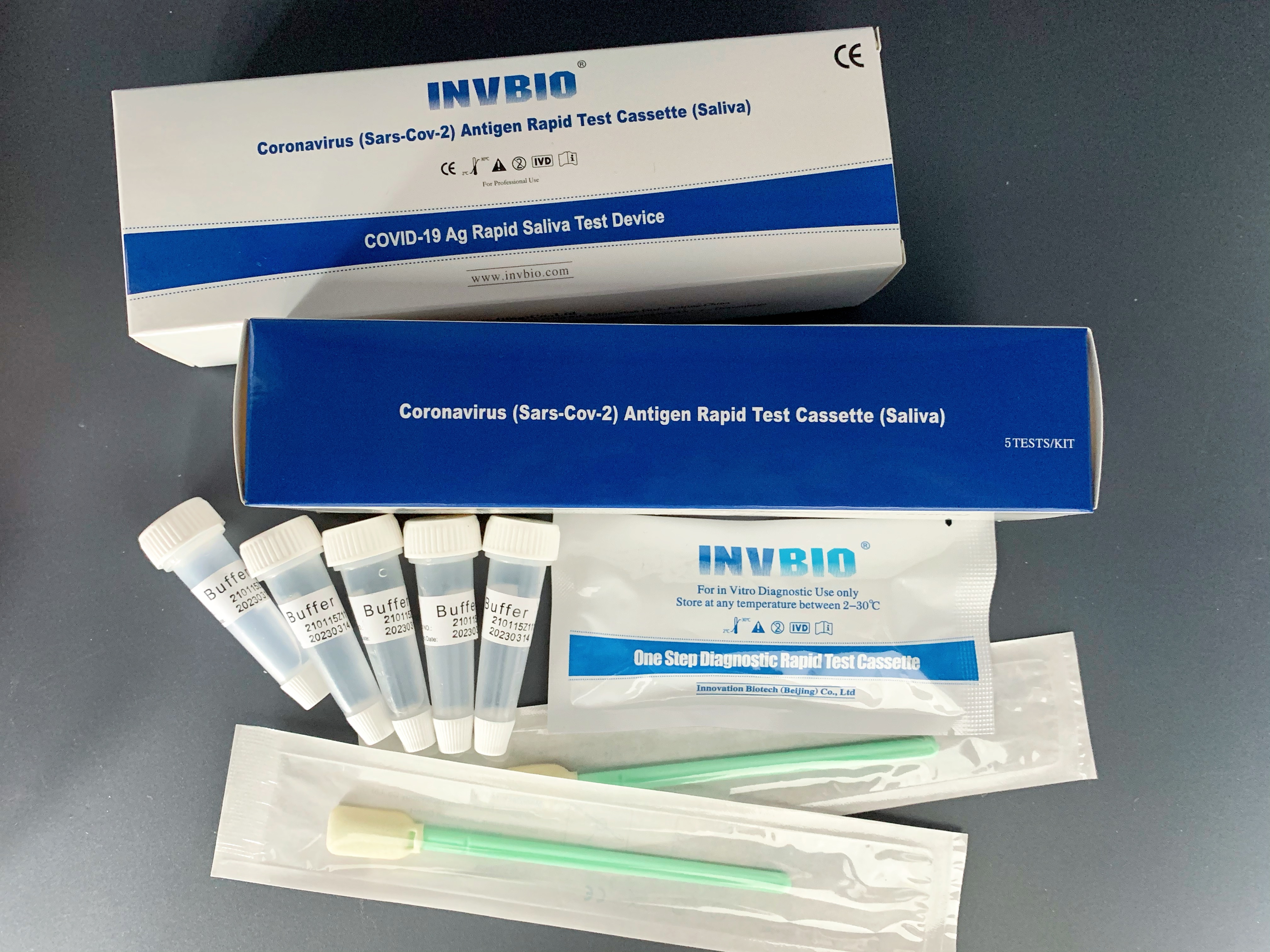
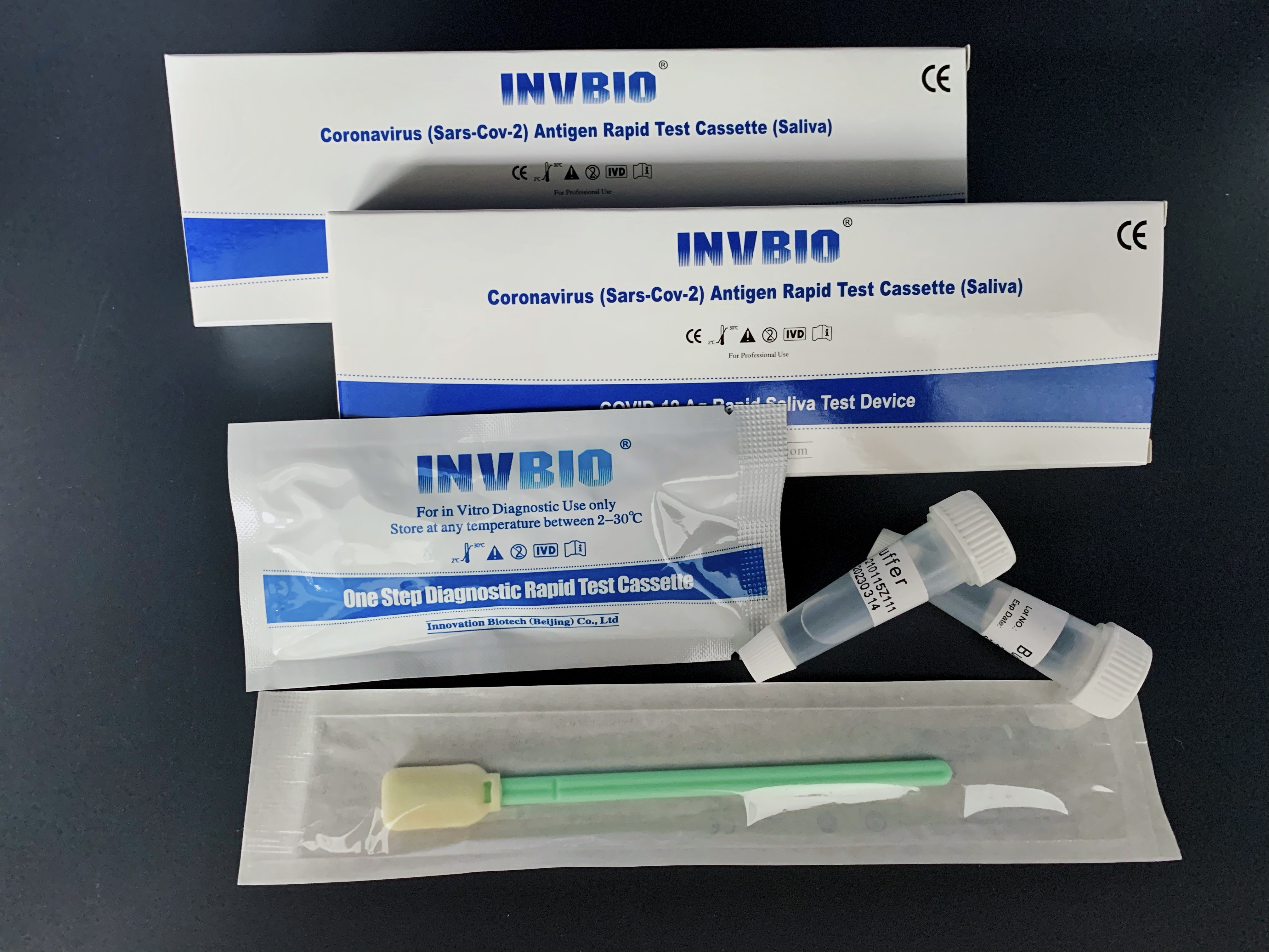
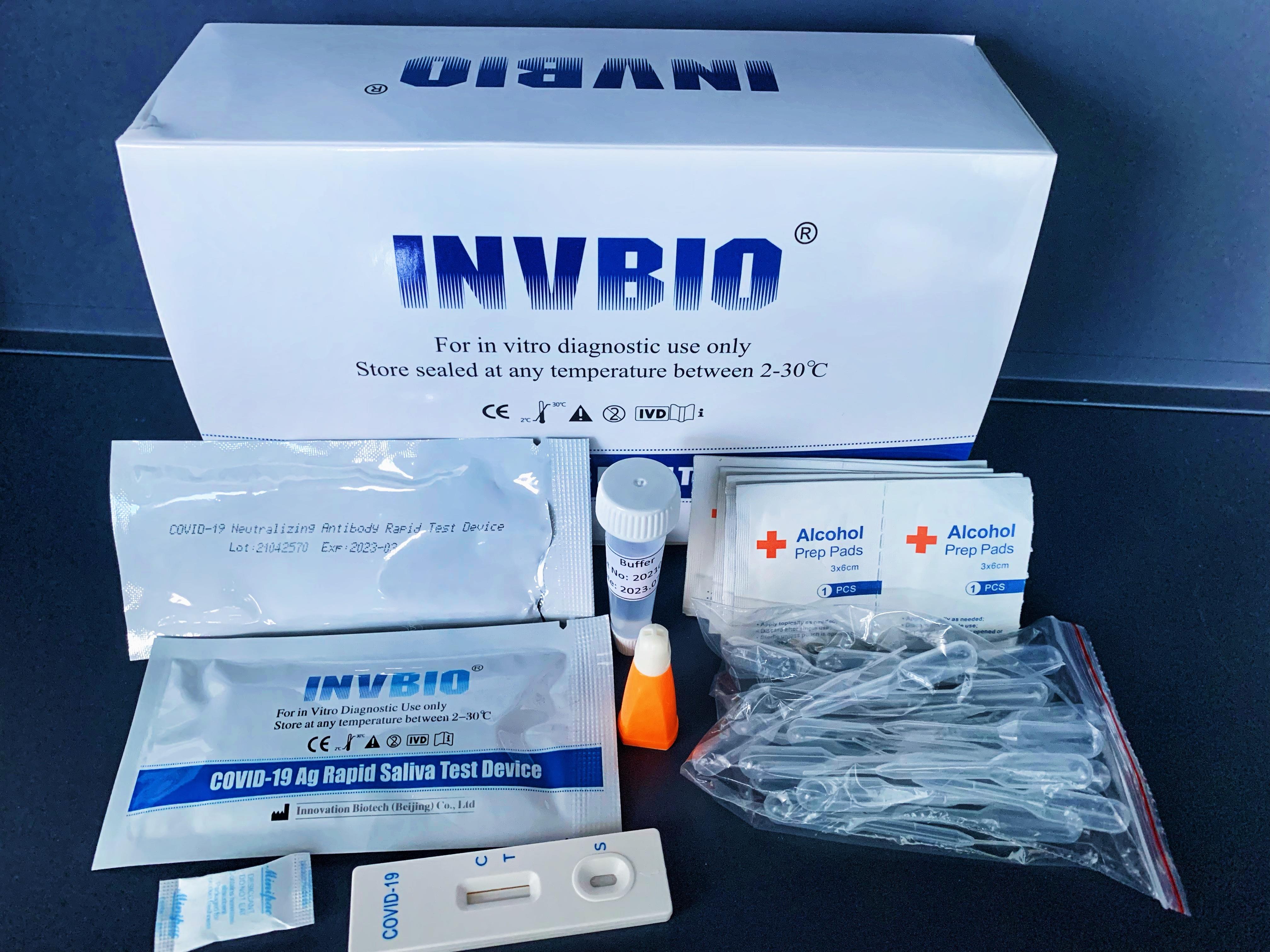
Covid -19 Ag saliva rapid test kit
Product Description
| Product name: | Covid -19 Ag saliva rapid test kit |
| Format: | Card |
| Specimen: | Saliva |
| Sensitivity: | 95.6% |
| Accuracy: | 98.21% |
| Specificity: | 99% |
| Packing | 25pcs/box; 5pcs/box; 1pcs/box |
| shelf life | 2years |
| Certificate: | CE , FSC |
Directions for Use
Innovation Biotech (Beijing) Co., Ltd. is a biotechnology company specializing in research, development and manufacturing of advanced medical in-vitro diagnostic (IVD) rapid test kits, medical and laboratory disposal products. We provide one step medical diagnostic rapid test kit based on our INVBIO brand, Product include Fertility Tests, Tumor markers, DOA drug of abuse test, Drug test cup, Urinalysis reagent strip, ELISA kit, Digital alcohol tester, urine analyzer, et.
OEM packaging is available, drug of abuse test, troponin I test, alcohol screening saliva test strips, urine strips, elisa kits, microscope slides
Our focus is to expand our markets internationally by forming strategic alliances and entering into partnerships with distributors worldwide. We have established an international reputation for excellence in the manufacturing of quality medical and laboratory products. In addition, we have obtained approval licenses ISO13485, FSC certificate, and most of our products get CE mark Our objective is the utmost satisfaction of our clients all around the world by supplying our quality and economical products


Mob/WhatsApp/WeChat/Skype: 0086 13356149018
Email: sales1@rapid-test-kit.com
Website: www.invbio.com
Q: What is your payment terms?
A: Payment terms: 100% TT before shipment
Q: Can you send samples?
A: Yes,SAMPLES can be sent for your evaluation.customer pay the freight charge
Q: Do you give any discount?
A: I'll surely try my best to help you get those by the best price and good service at the same time. We promise the best price upon same quality, and best quality upon same price.
Q: How to order?
A: Please tell us the model and quantity and package requests.
b. Proforma Invoice confirmed, the order will be arranged upon receipt of your payment.
c. Confirm and ship the goods.
d. We will help to track your goods until you receive them safely.


 Ordinary
Ordinary
 verified
verified
Business Type Manufacturer
Country / Region Beijing,China
Main Products Oral & Dental Equipment, In vitro Diagnosis Reagents, Blood Diagnosis Reagents, POCT Diagnostic Reagents, Tumor Marker Diagnostic Reagents, Home Health Equipment, Laboratory Equipment, Reagents & Chemicals
Main Markets
Brand : INVBIO
Min.Order : 1000 Piece(s)
Brand : INVBIO
Min.Order : 1000 Piece(s)
Brand : INVBIO
Min.Order : 1000 Piece(s)

Brand : INVBIO
Min.Order : 1000 Piece(s)

Brand : INVBIO
Min.Order : 1000 Piece(s)
brand name : INVBIO
certification :
fob price :
min.order : 1000 Piece(s)
warranty : 24 months
payment terms : T/T
Packaing : 20 test per box
Specification : 1000 boxes per carton
Trademark : INVBIO
Production Capacity :
place of origin : Beijing
Manag Certifica : CE,CFS
Covid -19 Ag saliva rapid test kit
Product Description
| Product name: | Covid -19 Ag saliva rapid test kit |
| Format: | Card |
| Specimen: | Saliva |
| Sensitivity: | 95.6% |
| Accuracy: | 98.21% |
| Specificity: | 99% |
| Packing | 25pcs/box; 5pcs/box; 1pcs/box |
| shelf life | 2years |
| Certificate: | CE , FSC |



Directions for Use
Innovation Biotech (Beijing) Co., Ltd. is a biotechnology company specializing in research, development and manufacturing of advanced medical in-vitro diagnostic (IVD) rapid test kits, medical and laboratory disposal products. We provide one step medical diagnostic rapid test kit based on our INVBIO brand, Product include Fertility Tests, Tumor markers, DOA drug of abuse test, Drug test cup, Urinalysis reagent strip, ELISA kit, Digital alcohol tester, urine analyzer, et.
OEM packaging is available, drug of abuse test, troponin I test, alcohol screening saliva test strips, urine strips, elisa kits, microscope slides
Our focus is to expand our markets internationally by forming strategic alliances and entering into partnerships with distributors worldwide. We have established an international reputation for excellence in the manufacturing of quality medical and laboratory products. In addition, we have obtained approval licenses ISO13485, FSC certificate, and most of our products get CE mark Our objective is the utmost satisfaction of our clients all around the world by supplying our quality and economical products


Mob/WhatsApp/WeChat/Skype: 0086 13356149018
Email: sales1@rapid-test-kit.com
Website: www.invbio.com
Q: What is your payment terms?
A: Payment terms: 100% TT before shipment
Q: Can you send samples?
A: Yes,SAMPLES can be sent for your evaluation.customer pay the freight charge
Q: Do you give any discount?
A: I'll surely try my best to help you get those by the best price and good service at the same time. We promise the best price upon same quality, and best quality upon same price.
Q: How to order?
A: Please tell us the model and quantity and package requests.
b. Proforma Invoice confirmed, the order will be arranged upon receipt of your payment.
c. Confirm and ship the goods.
d. We will help to track your goods until you receive them safely.

SARS-Cov-2 COVID-19 Neutralizing Antibody Rapid Test

Elisa Test Kits

DOA drug of abuse test multi drug test panel Drug Combination Test 12 in 1, 10 in 1, 6 in 1, 5 in 1, 3 in 1, 2 in 1

Novel COVID-19 Ag Nasal Test (swab)

INVBIO Covid 19 Rapid Test Kit 96.8% Accuracy Igg Igm Antibody Fast Detection Kit