

FOB Price : Get a Price/Quote
Min.Order : 1350 Piece(s)
Certification : CE
Brand Name : INVBIO
Payment Terms : T/T
brand name : INVBIO
certification : CE
min.order : 1350 Piece(s)
warranty : 24 months
payment terms : T/T
Packaging : 1 test per box
Specification : 270 boxes per carton
place of origin : Beijing
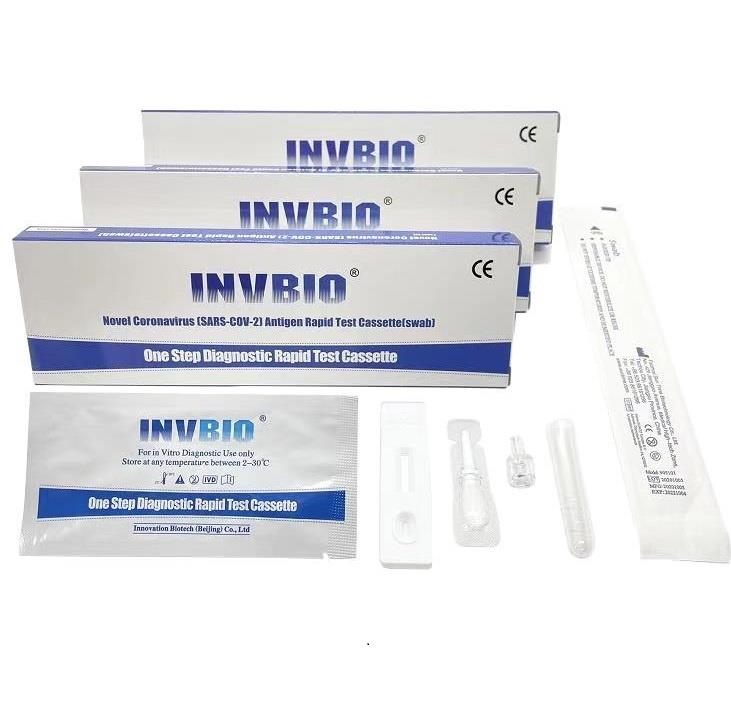
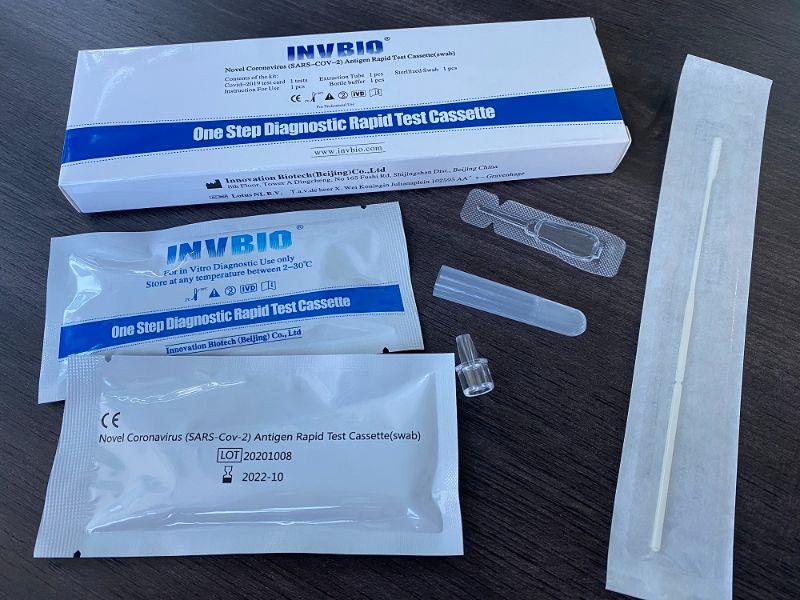
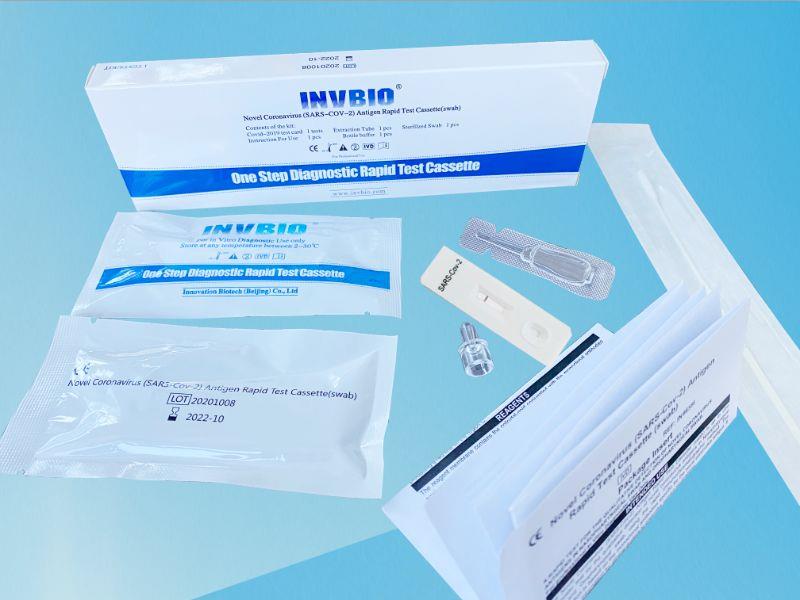
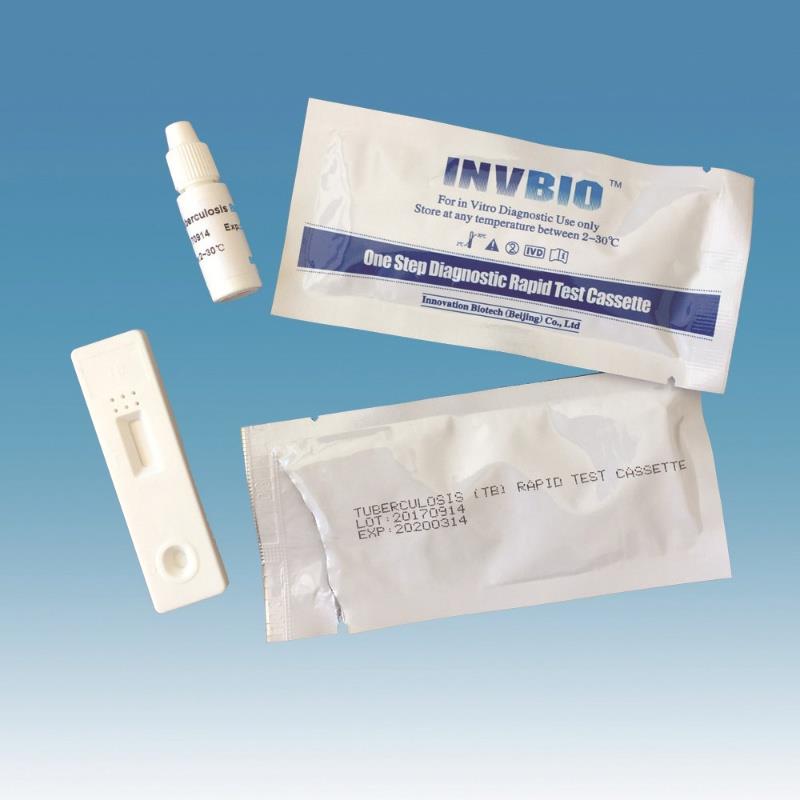
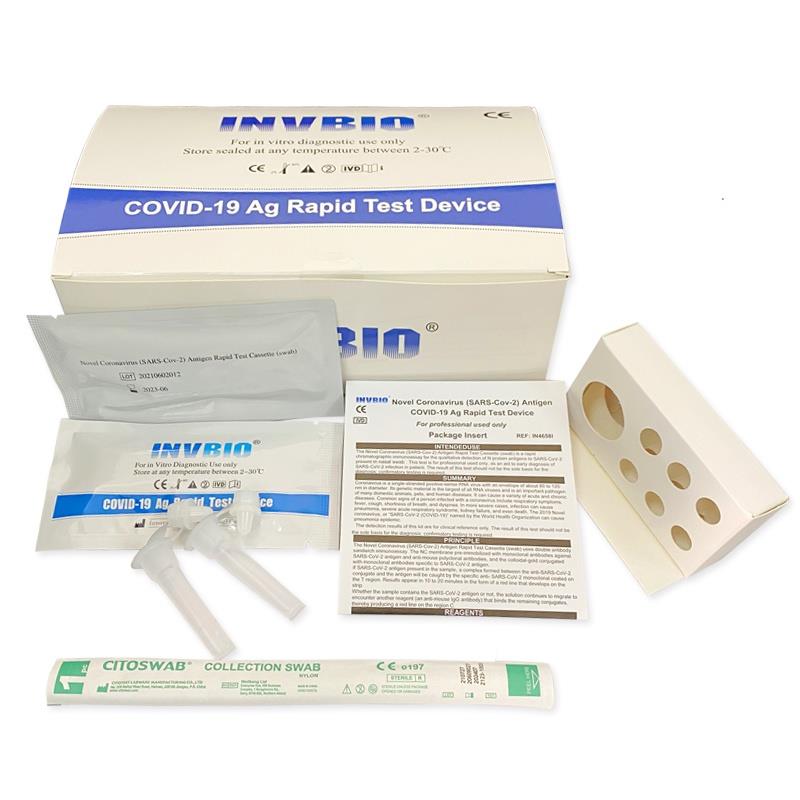
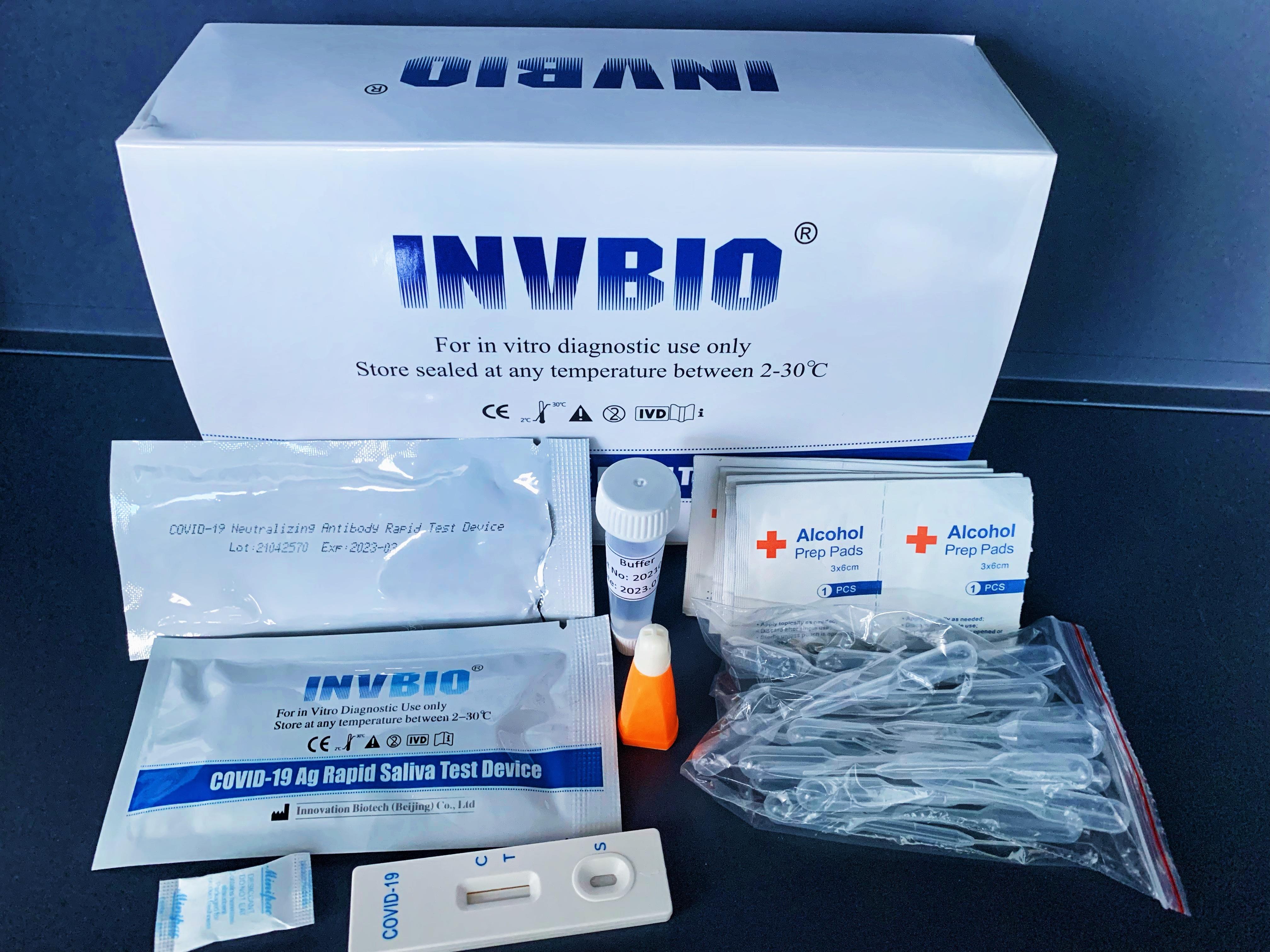
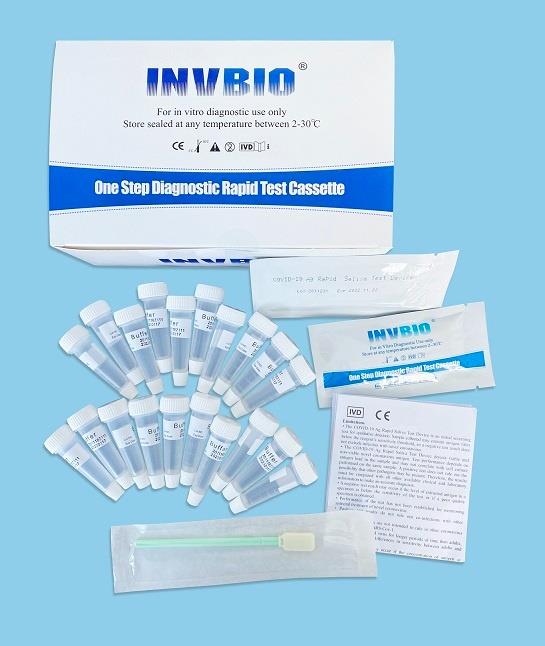
The Novel Coronavirus (SARS-Cov-2) Antigen Rapid Test Cassette (swab) is a rapid chromatographic immunoassay for the qualitative detection of N protein antigens to SARS-CoV-2 present in nasal swab . This test is for professional used only, as an aid to early diagnosis of SARS-CoV-2 infection in patient. The result of this test should not be the sole basis for the diagnosis; confifirmatory testing is required.
| Product name: | Covid -19 Antigen nasal swab rapid test kit |
| Format: | Card |
| Specimen: | Swab |
| Sensitivity: | 95.6% |
| Accuracy: | >99% |
| Specificity: | 98.21% |
| Packing | 25pcs/box; 5pcs/box; 1pcs/box |
| shelf life | 2years |
| Certificate: | CE , FSC |



Q: What is your payment terms?
A: Payment terms: 100% TT before shipment
Q: Can you send samples?
A: Yes,SAMPLES can be sent for your evaluation.customer pay the freight charge
Q: Do you give any discount?
A: I'll surely try my best to help you get those by the best price and good service at the same time. We promise the best price upon same quality, and best quality upon same price.
Q: How to order?
A: Please tell us the model and quantity and package requests.
b. Proforma Invoice confirmed, the order will be arranged upon receipt of your payment.
c. Confirm and ship the goods.
d. We will help to track your goods until you receive them safely.

 Ordinary
Ordinary
 verified
verified
Business Type Manufacturer
Country / Region Beijing,China
Main Products Oral & Dental Equipment, In vitro Diagnosis Reagents, Blood Diagnosis Reagents, POCT Diagnostic Reagents, Tumor Marker Diagnostic Reagents, Home Health Equipment, Laboratory Equipment, Reagents & Chemicals
Main Markets

Brand : INVBIO
Min.Order : 1000 Piece(s)
Brand : INVBIO
Min.Order : 1000 Piece(s)
Brand : INVBIO
Min.Order : 1000 Piece(s)
Brand : INVBIO
Min.Order : 1000 Piece(s)

Brand : INVBIO
Min.Order : 1000 Piece(s)
brand name : INVBIO
certification :
fob price :
min.order : 1350 Piece(s)
warranty : 24 months
payment terms : T/T
Packaing : 1 test per box
Specification : 270 boxes per carton
Trademark : INVBIO
Production Capacity :
place of origin : Beijing
Manag Certifica : CE
The Novel Coronavirus (SARS-Cov-2) Antigen Rapid Test Cassette (swab) is a rapid chromatographic immunoassay for the qualitative detection of N protein antigens to SARS-CoV-2 present in nasal swab . This test is for professional used only, as an aid to early diagnosis of SARS-CoV-2 infection in patient. The result of this test should not be the sole basis for the diagnosis; confifirmatory testing is required.
| Product name: | Covid -19 Antigen nasal swab rapid test kit |
| Format: | Card |
| Specimen: | Swab |
| Sensitivity: | 95.6% |
| Accuracy: | >99% |
| Specificity: | 98.21% |
| Packing | 25pcs/box; 5pcs/box; 1pcs/box |
| shelf life | 2years |
| Certificate: | CE , FSC |



Q: What is your payment terms?
A: Payment terms: 100% TT before shipment
Q: Can you send samples?
A: Yes,SAMPLES can be sent for your evaluation.customer pay the freight charge
Q: Do you give any discount?
A: I'll surely try my best to help you get those by the best price and good service at the same time. We promise the best price upon same quality, and best quality upon same price.
Q: How to order?
A: Please tell us the model and quantity and package requests.
b. Proforma Invoice confirmed, the order will be arranged upon receipt of your payment.
c. Confirm and ship the goods.
d. We will help to track your goods until you receive them safely.