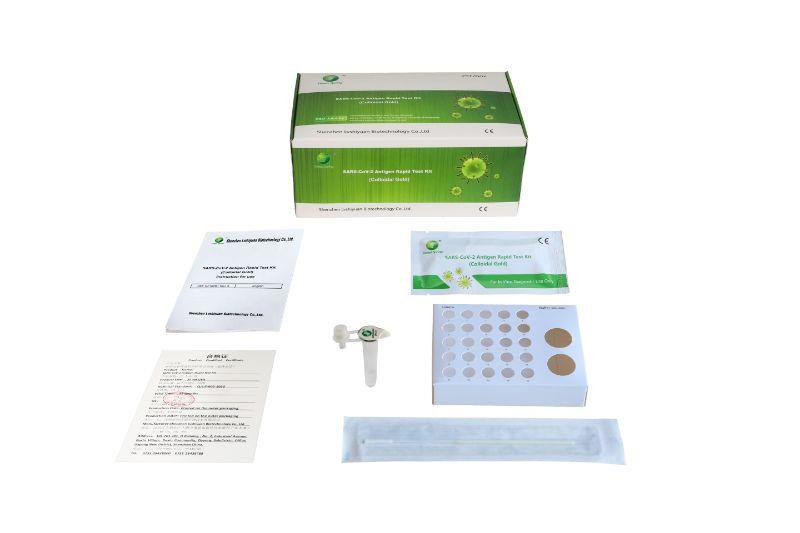
Professional Tests Version:
Component:
1.Test cassette in foil pouch with desiccant
2.Sterile Swab
3.Pre-filled extraction tube with buffer, seal cap and dropper tip cap Tube stand
4.Package insert
Storage and Stability:
Store the kits in the sealed foil pouch at room temperature or refrigerated (2- 30 °C). The kit is stable until the expiry date. The Test cassettes must be stored in the sealed foil pouch until use. Do not freeze. Do not use after expiration date. Protect from sun, moisture and heat.
Specimen collection and requirements
Correct sample collection is the most important step. Select one of the 4 methods and then proceed with the test procedure.
1) Anterio-nasal swab (nose in front)
Make sure to collect sufficient nasal secretions with the swab. It is recommended to blow your nose first.
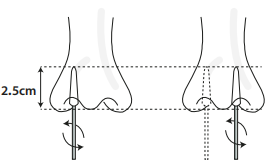
1. Carefully insert the swab into the patient's nostril. The swab tip should be inserted up to 2.5 cm deep from the edge of the nostril.
2. Swab along the mucosa in the nostril to ensure that both mucus and cells are collected.
3. Remove the swab from the nostril while gently rotating it between your fingers.
2) Nasopharyngeal swab (nose-throat).
1. Tilt the patient's head slightly backwards. Hold the swab like a pen and insert it through the nostril parallel to the palate.
2. While inserting, gently rub and roll the swab. Once you feel the throat resistance, stop and let the swab absorb secretion.
3. Slowly and gently remove the swab outward while gently rotating it between your fingers.
3) Oropharyngeal swab (throat)
1. Have the patient open his/her mouth wide and make "Ah" sounds, exposing the pharyngeal tonsils on both sides.
2. Hold the swab firmly and wipe back and forth on the pharyngeal tonsils on both sides at least three times per side with moderate force. Do not touch the palate, tongue, teeth or gums.
3. Remove the swab while gently rotating it between your fingers.
4) Saliva (Lollipop Test )
Be aware that incorrect results may occur if saliva is not collected properly.
1. Press the tip of the tongue against the lower root of the jaw. Cough deeply. Make the sound of "kuuua" to concentrate the saliva.
2. Place the swab on the tongue for at least 10 seconds and rotateit at least 3 times to completely absorb the saliva.
For best results, the nasopharyngeal (nose-to-throat) method is recommended.
Test Procedure: After sampling, perform the test as follows:
1. Tear off the seal of the extraction buffer tube.
2. Insert the swab into the buffer tube and dip it up and down for at least 10 seconds so that the sample mixes with the extraction liquid.
3. Remove the swab while squeezing the sides of the tube to extract the liquid from the swab. Make sure that no contents splash out of the tube.
4. Close the tube with the dropper tip. Add 3 drops (approx. 100μL)to the sample well of the test kit via the dropper tip.
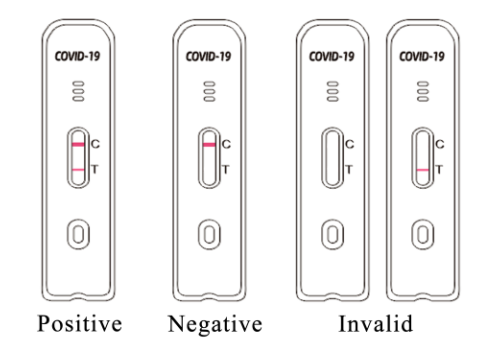
5. Interpret the test results after 15 minutes. Do not interpret the results later than 20 minutes.
Performance Characteristics:
Clinical performance
The clinical performance of the Green Spring® SARS-CoV-2 rapid antigen test was determined in prospective, randomized, single-blind studies. A total of 365 nasopharyngeal specimens from symptomatic and asymptomatic patients were collected within 5 days of the onset of initial symptoms. The performance of the kit was compared with the results of a commercially available molecular test. The test was found to have a sensitivity of 96.77% and a specificity of 100.00%. The accuracy is 98.63%. A nasopharyngeal swab is used in the PCR comparisons.
For the anterior nasal swab method, 298 specimens were collected. A sensitivity of 96.25% and a specificity of 100.00% were determined for the test. The accuracy is 97.99%. A nasopharyngeal swab is used in the PCR comparisons.
Cross-reactivity
No cross-reactivity with potentially cross-reactive substances other than SARS coronavirus were observed.
Self Testing Vision With CE1434:
Component:
| Component |
Description |
Specification |
1 test/kit
Ref.GF102BS1 |
5 test/kit
Ref.GF102BS5 |
10 test/kit
Ref.GF102BS10 |
25 test/kit
Ref.GF102BS25 |
| Test cassette |
Foil ouched test device containing one reactive strip. |
1 |
5 |
10 |
25 |
| Sterile swab |
For sample collection and transfer. |
1 |
5 |
10 |
25 |
| Inside page |
Instructions for use. |
1 |
1 |
1 |
1 |
| Extraction buffer |
Dissolve the sample |
1 |
5 |
10 |
25 |
| The certificate of conformity |
|
1 |
1 |
1 |
1 |
| Packages with holes |
Be used as tube stand (optional). |
1 |
1 |
1 |
1 |
Storage and Disposal
Store at room temperature (2-30°C or 35.6-86 T).
The test cassettes must be stored in the sealed foil pouch before use.
Do not use after the expiration date.
Waste from used tests should be disposed according to the local regulations.
Preparation
Read the instructions for use carefully before performing the test. Blow your nose and then wash your hands thoroughly with soap or disinfect them with disinfectant. Keep the test cassette and components at room temperature (15°C to 30°C) before performing the test. Place all the materials supplied on a clean, dry and flat surface.
Sampling in the nose
Please follow the instructions on the next page step by step for sampling test.
Test Procedure
1.Blow your nose. Wash or disinfect your hands. Remove the test cassette by tearing open the foil pouch and place it in front of you.
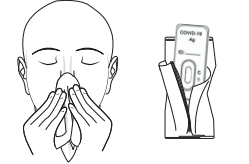
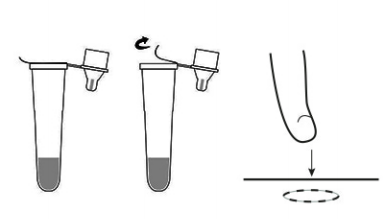
2. Tear off the seal of extraction buffer tube, press in the perforated hole on the top of the packaging and use the hole as a tube stand.
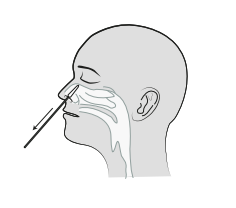
3.Remove the swab. Do not touch the sterile tip of the swab. Instead, grasp the swab by the handle. Insert the swab about 2.5 cm deep into your nostril until you feel resistance.
4.Collect sample from the left and right nostril with the same swab: rub the swab against the inner wall of your nose and turn it at least 5 times to make sure you collect a sufficient amount of
sample. Repeat the procedures in the other nostril.
Note: Children (at least 2 years old) younger than 15 years old, and people who are unable to perform the test themselves including the elderly and the sick should be tested by another adult. To sample a child, insert the swab into of one of their nostrils until you feel some resistance (about 2 cm). Rotate the swab 5 times against the nasal wall. Remove the swab and insert the same swab into the other nostril, repeat the sampling process. Do not continue the test if the child feels any pain.
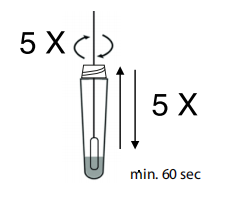
5.Dip the swab into the tube and ensure that it is thoroughly mixed with the extraction liquid by agitating it and dipping it up and down at least 5 times. Allow the swab to soak for one minute.
6.Slowly pull the swab out of the tube while gently squeezing the sides of the tube to keep as much liquid in the tube as possible.
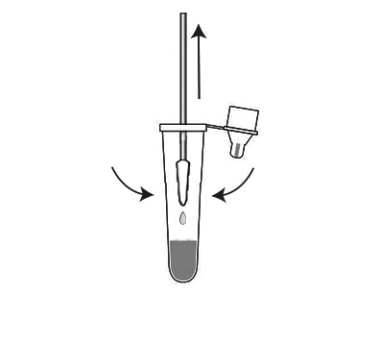
7.Place the dropper tip firmly on the extraction buffer tube and mix the liquid thoroughly.
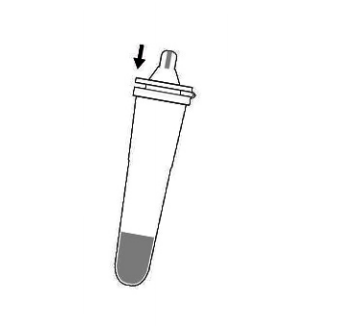
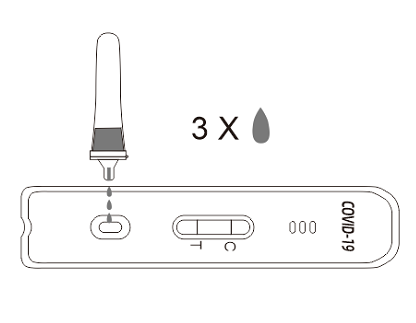
8.Drop 3 drops into the sample well (S) on the test card.
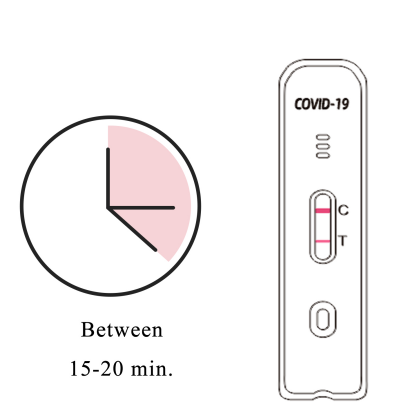
9.Interpret the test result between 15-20 minutes. The result after 20 minutes is invalid.
Cross Reactivity and Microbial Interference
The cross-reactivity with the following microorganisms was examined. Samples that tested positive for the following microorganisms were negative when tested with the SARS-CoV-2 Antigen Rapid Test (Colloidal Gold). The microbial interference study evaluated whether microorganisms possibly contained in clinical samples interfere with the detection capability of the kit which may lead to false negative results. Each microorganism was tested in the presence of a fabricated SARS-CoV-2 positive sample. No cross-reactivity or interference with the microorganisms listed in the table below was found.
| No. |
Microorganism |
Final Test Concentration |
| 1 |
SARS-Cov recombinant protein |
2.5 mg/mL |
| 2 |
MERS-Cov recombinant protein |
2.07 mg/mL |
| 3 |
Adenovirus (Type 1) |
1.0×107 pfu/mL |
| 4 |
Adenovirus (Type 3) |
1.0×107 pfu/mL |
| 5 |
Coronavirus(229E) |
1.0×107 pfu/mL |
| 6 |
Coronavirus (HKU1) |
1.0×107 pfu/mL |
| 7 |
Coronavirus (NL63) |
1.0×107 pfu/mL |
| 8 |
Coronavirus (OC43) |
1.0×107 pfu/mL |
| 9 |
Influenza A Seasonal H1N1 |
1.0×107 pfu/mL |
| 10 |
In?uenza B Yamagata |
1.0×107 pfu/mL |
| 11 |
Legionella pneumonia |
1.0×107 pfu/mL |
| 12 |
MERS |
1.0×107 pfu/mL |
| 13 |
Mycobacterium tuberculosis |
1.0×107 pfu/mL |
| 14 |
Mycoplasma pneumoniae |
1.0×107 pfu/mL |
| 15 |
Parain?uenza virus (Type 1) |
1.0×107 pfu/mL |
| 16 |
Respiratory syncytial virus |
1.0×107 pfu/mL |
| 17 |
Rhinovirus(Group A) |
1.0×107 pfu/mL |
| 18 |
Rhinovirus(Group B) |
1.0×107 pfu/mL |
Interference
The following interfering substances have no impact on SARS-CoV-2 Antigen Rapid Test (Colloidal Gold).
| No. |
Interfering Substance |
Final Test Concentration |
| 1 |
Mucin |
0.54% |
| 2 |
Menthol |
1.4 mg/mL |
| 3 |
Whole Blood |
5% |
| 4 |
Triamcinolone acetonide |
1 ng/mL |
| 5 |
Tobramycin |
5 μg/mL |
| 6 |
Levofloxacin |
1.5 μg/mL |
| 7 |
Mupirocin |
12 mg/mL |
| 8 |
Oxymetazoline |
9%v/v |
| 9 |
Nasal Spary |
16%v/v |
| 10 |
Dexamethasone |
0.5 μg/mL |













 Ordinary
Ordinary
 verified
verified









