


FOB Price : Get a Price/Quote
Min.Order :
Certification : CFDA,CE
Brand Name : Scenker
Payment Terms : T/T,EXW
brand name : Scenker
certification : CFDA,CE
min.order :
warranty :
payment terms : T/T,EXW
Packaging :
Specification :
place of origin : Shandong
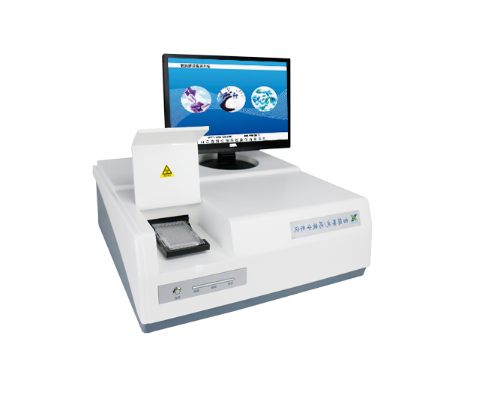
Product Details
Microbial ID/AST System
Product Overview
• Based on latest microbial classification scheme, the product provides rapid and accurate species-level identification of clinically important pathogenic bacteria and yeasts.
• Strong software functions not only help clinicians to select the best antibiotics, but also provide the greatest help for the epidemiological investigation, drug resistance trend analysis and hospital infection management.

Product Features
• Detection Principle
—Colorimetric method for microorganism identification and turbidity method for antibiotic susceptibility testing (AST), no need any additional test.
• Detection Method
—Photoelectric micro-well by micro-well detection with 8 road detection unit, no interference between the micro-wells, test results without manual review.
• AST
—The tested antibiotics selected according to CLSI standards and the "Guidelines for the Use of Antimicrobial Agents" cover the clinical commonly used antibiotics. The activities of antibiotics have been strictly calibrated and the AST results can be grouped reported according to the CLSI standards (A, B, C, O, U, etc.).
• Expert System
—Expert system provids validation of identification and AST results and give an accurate phenotype profile of the bacterial resistance menchanism(s) for each isolate tested to help clinicians select the most appropriate antibiotic treatment.
• ID/AST results can be provided in 18-24 hours, as little as 4 to 6 hours
• Hospital Infection Analysis
—Report of hospital infection surveillance is designed compliance with the GB15982 standard and information of nosocomial infection and drug resistance rate changes can be reported.
• Quality Control
—Powerful QC function can meet the quality control requirement of any size lab.
• Statistical Analysis
—Software support query statistics according to the information of isolates, inspection departments and specimens samples
• Connect with users’ LIS and transfer data to WHONET



 Ordinary
Ordinary
 verified
verified
Business Type Manufacturer
Country / Region Shandong,China
Main Products In vitro Diagnosis Consumables, In vitro Diagnosis Reagents, Laboratory Devices, Reagents & Chemicals
Main Markets
brand name : Scenker
certification :
fob price :
min.order :
warranty :
payment terms : T/T,EXW
Packaing :
Specification :
Trademark :
Production Capacity :
place of origin : Shandong
Manag Certifica : CFDA,CE
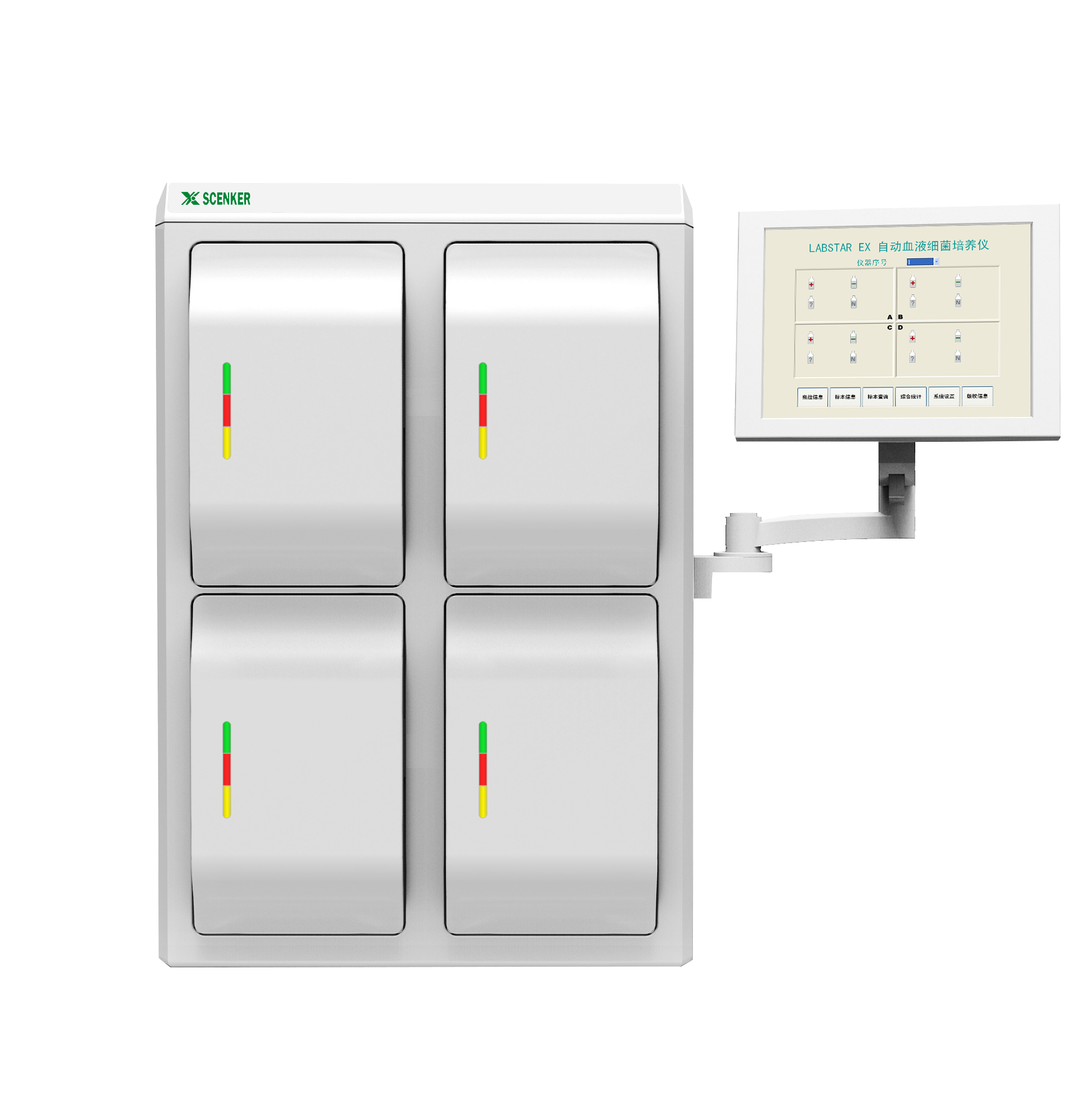
Product Details
Microbial ID/AST System
Product Overview
• Based on latest microbial classification scheme, the product provides rapid and accurate species-level identification of clinically important pathogenic bacteria and yeasts.
• Strong software functions not only help clinicians to select the best antibiotics, but also provide the greatest help for the epidemiological investigation, drug resistance trend analysis and hospital infection management.

Product Features
• Detection Principle
—Colorimetric method for microorganism identification and turbidity method for antibiotic susceptibility testing (AST), no need any additional test.
• Detection Method
—Photoelectric micro-well by micro-well detection with 8 road detection unit, no interference between the micro-wells, test results without manual review.
• AST
—The tested antibiotics selected according to CLSI standards and the "Guidelines for the Use of Antimicrobial Agents" cover the clinical commonly used antibiotics. The activities of antibiotics have been strictly calibrated and the AST results can be grouped reported according to the CLSI standards (A, B, C, O, U, etc.).
• Expert System
—Expert system provids validation of identification and AST results and give an accurate phenotype profile of the bacterial resistance menchanism(s) for each isolate tested to help clinicians select the most appropriate antibiotic treatment.
• ID/AST results can be provided in 18-24 hours, as little as 4 to 6 hours
• Hospital Infection Analysis
—Report of hospital infection surveillance is designed compliance with the GB15982 standard and information of nosocomial infection and drug resistance rate changes can be reported.
• Quality Control
—Powerful QC function can meet the quality control requirement of any size lab.
• Statistical Analysis
—Software support query statistics according to the information of isolates, inspection departments and specimens samples
• Connect with users’ LIS and transfer data to WHONET
